BREAKING: Methane-Reducing Feed Additive Trialled in Arla Dairy Farms
On November 26th, Arla Foods Ltd. announced via social media their collaboration with major UK supermarkets like Tesco, Aldi, and Morrisons to trial Bovaer, a feed additive, aiming to reduce methane emissions in the UK.
The following is a transcript of the video commentary.
“The latest innovation we're looking at is Bovaer. Bovaer is a feed additive developed by Dutch company DSM that allows farmers to reduce enteric methane emissions from our dairy herds. Enteric methane being what the cows produce during their natural digestive process. Today, we've been working with Arla and DSM as part of the Bovaer set-up process, learning about what's involved and how we can make the pilot as successful as possible.
So the reason we quite excited about Bovaer, it's not just some fairy dust that somebody thinks might make some improvements but it's been really rigorously and extensively tested. Trials like these are really important to help reduce our carbon footprint.”
Arla Foods is a major player in the UK dairy market. It supplies milk to retailers and produces brands such as Lurpak, Anchor, Cravendale, Lactofree, and Castello.
What is Bovaer?
Bovaer is a feed additive designed to reduce enteric methane emissions (belching/farting) from dairy and beef cattle. It has been authorized for use in over 50 countries, including Australia, Brazil, Canada, the US and EU countries. In April 2024, it was authorized in the UK.
Bovaer is a product owned by Netherlands-based, DSM-Firmenich, a company whose work “supports the development of a more sustainable global animal production industry” and is part of the fake-food and synthetic biology sector.
DSM’s website states: “Bovaer® contributes to a significant and immediate reduction of the environmental footprint of beef and dairy products. Just a quarter teaspoon per cow per day reduces methane emissions from dairy cattle by 30% and up to 45% for beef cattle, on average.”
According to the Food and Agriculture Organization of the United Nations, livestock produce 12% of global greenhouse gas (GHG) emissions. Efforts to combat "climate change" have long focused on livestock.
In January 2023, Bill Gates’ investment firm, Breakthrough Energy Ventures (BEV), led a $12 million seed funding round into Rumin8, a rival Australian start-up focused on developing feed supplements to reduce methane emissions from livestock.
Arla’s announcement caused quite a stir on social media with many accounts calling for a boycott of their products.
What is the active ingredient in Bovaer?
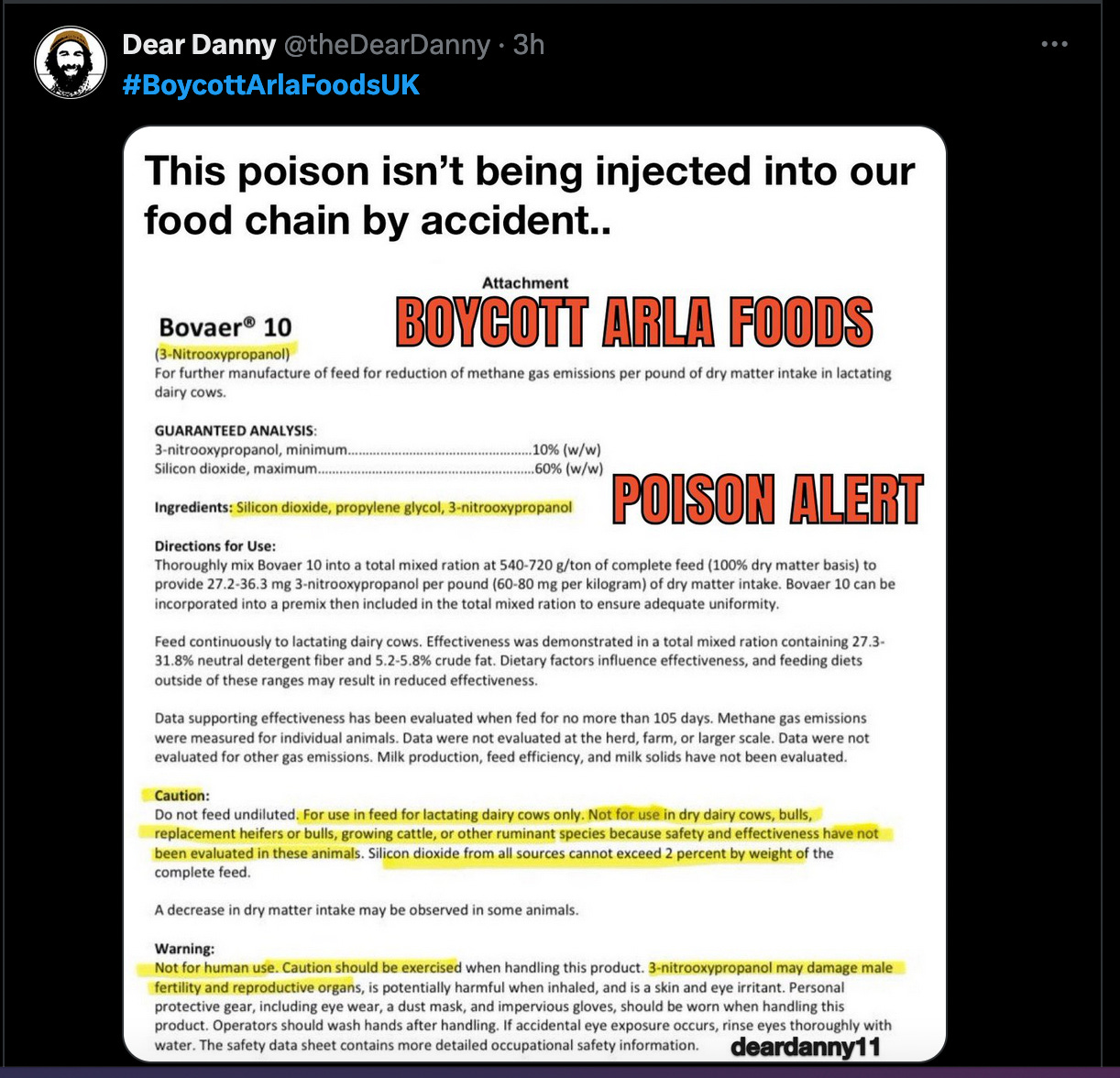
3-nitrooxypropanol (3-NOP) is the active ingredient in Bovaer. Other ingredients include silicon dioxide and propylene glycol. 3-NOP inhibits the enzyme methyl-coenzyme M reductase in the rumen (the first stomach compartment in ruminant animals)- preventing the final step in methane production.
Is it safe?
It has been claimed that the additive has no side effects. However, while looking through some of the available reports, certain anomalies stand out.
According to the FDA letter addressed to Elanco US, Inc, (which has an agreement with DSM-Firmenich to market Bovaer), it states: “Bovaer® 10 is an article (other than food) intended to affect the structure or any function of the body of an animal, and therefore it is a drug. However, the Center for Veterinary Medicine (CVM) has considered whether it intends to exercise enforcement discretion with regard to certain requirements applicable to animal drugs for Bovaer® 10 – including requirements regarding new animal drug approval, pharmaceutical current Good Manufacturing Practice, adverse event reporting, labeling, drug establishment registration and drug product listing. CVM has considered whether refraining from enforcement of these requirements at this time would be appropriate based on whether Bovaer® 10 poses a low risk to humans and animals and whether the data show that the product has the intended effect. Based on a review of your data and the characteristics of your product, FDA has no questions at this time regarding whether Bovaer® 10 will achieve its intended effect and is expected to pose low risk to humans or animals under the conditions of its intended use.”
The FDA's Center for Veterinary Medicine (CVM) seems to be sending mixed messages. Although Bovaer is officially classified as a drug, the CVM appears to have decided not to enforce certain drug regulations, including the obligation for adverse event reporting. This decision is based on the belief that Bovaer "is expected to pose low risk to humans or animals."
Paradoxically, the letter includes an attachment with the following troubling statements.
The FSA and FSS safety assessment report
Turning to the safety assessment report for Bovaer, prepared by the UK’s Food Standards Agency (FSA) and Food Standards Scotland (FSS), which was reviewed by Animal Feed and Feed Additives Joint Expert Group (AFFAJEG) and the Advisory Committee on Animal Feedingstuffs (ACAF).
According to ACAF: “the additive can be considered safe for consumers” yet this conclusion is seemingly contradicted by the statement: “The additive should be considered corrosive to the eyes, a skin irritant and potentially harmful by inhalation.”
Notably, the assessment states: “In their first evaluation, members observed that no analysis was performed on the final product to screen for dioxins and heavy metals.” This was followed by a request made by AFFAJEG for the applicant to provide an analysis of impurities in the final product.
In the two tolerance studies presented in DSM’s application in relation to safety for the target species: “Study 1 used only 4 cows per group, which were given 0, 100, 500 and 1000 mg 3-NOP/kg feed DM for 90 days. The highest dose showed a reduced intake of feed and a reduced heart weight.”
Study 2 was larger and used 20 cows per group, “which were given doses of 3-NOP of 0, 80, 100, or 200 mg/kg feed DM for 56 days.” The highest dose (the 200 mg dose group), negative results were identified, which included “decreased ovary size, decreased serum activities of ALT (alanine aminotransferase) and LDH (lactate dehydrogenase), and reduced feed and water intake.”
The report went on state “The decrease in ovarian size was not accompanied by histopathological change and it was concluded that it should not be considered an adverse effect of the study at the 200 mg/kg dose…The Group concluded that the additive could be considered safe at a dose of 200 mg/kg and that a margin of tolerance of 2 could be established.”
In relation to safety studies for the consumer, a 2-year carcinogenicity study in Wistar rats showed “mesenchymal cell tumours were reported in 4 out of 49 females at the top dose of 300 mg/kg bw/day of 3-NOP given orally. Based on these results, the original study report concluded there was evidence of carcinogenicity in female rats.”
Curiously, this conclusion was changed after an independent group was brought in to “reanalyse the study’s slides.” This group concluded that “mesenchymal cell tumours were present in 3 out of 49 females at the top dose group, which was no longer statistically significantly different from the control group.”
The AFFAJEG concluded that at the higher dose levels (300 mg/kg/day in females), the additive has the potential to cause mesenchymal cell hyperplasia and benign tumours. However, due to the “absence of malignant tumours and genotoxicity, it was concluded that the additive is not carcinogenic at the recommended inclusion rate and benign tumours occurred only above the NOAEL.”
In the genotoxocity studies testing whether 3-NOP is toxic to genes, it was noted “that positive results occurred in Chinese hamster V79 cells, with negative results in a study using human peripheral blood lymphocytes and an equivocal result in a study using TK6 cells..” Positive results indicate that the substance tested has the potential to cause genetic damage.
In the second study, the results were negative except for males dosed at the top dose and sacrificed at 24 hours, in which micronuclei were statistically significantly increased compared to the negative control.
Alarmingly, the report went on to state: “Based on the OECD guidance on establishing the biological relevance of a result in this assay, which is neither clearly positive nor clearly negative, AFFAJEG members recognised the requirement for external expert judgement. An external consultant, contracted by the applicant, concluded that the apparent increase in micronuclei may have been an artifact due to the Giemsa-based stain that was used, to which Group experts agreed. The Group concluded that 3-NOP is non-genotoxic in vivo.”
This raises alarm bells considering it was an external consultant, paid for by the applicant (DSM), who attributed another cause for the increase in micronuclei- the stain used- not 3-NOP, the active ingredient in DSM’s product, Bovaer.
I will be updating this report as more information is attained.
In an era where information is often manipulated, your support for independent investigative journalism is not just appreciated—it's vital! My work has always been about unearthing the uncomfortable truths. By upgrading to a paid subscription for less than the cost of a coffee each month, you can help keep the flame of investigative journalism alive!





Leave the livestock as God created , this is ridiculous
I see M&S are in on this too. I’ve just emailed Waitrose to ask them to specify which products they sell will contain this as I do not wish to consume any of them and told them I’ll want to post the list on social media. We need a central space to go and check which products are ok to consume and then for that to go viral!